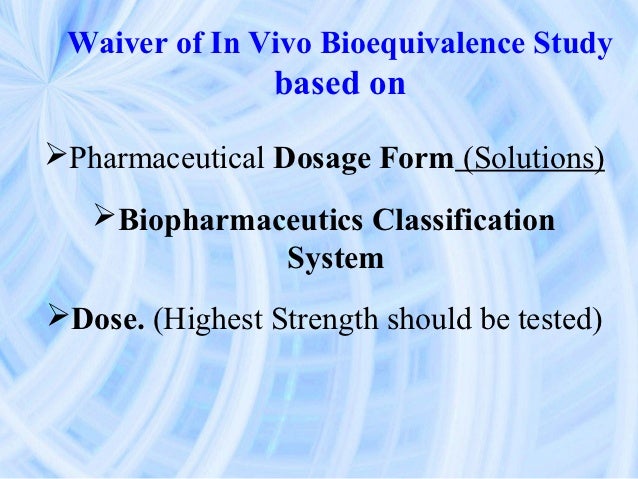
Bcs Pharmaceuticals
Classification of orally administered drugs on the World Health Organization model list of essential medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm. Bcs Pharma Corporation was founded in 2009, and is located at 7265 Oxford Way in Commerce. The Biopharmaceutics Classification System is a system to differentiate the drugs on the basis of their solubility and permeability. [1] This system restricts the prediction using the parameters solubility and intestinal permeability.
• • 2.9k Downloads • Abstract The Biopharmaceutical Classification System (BCS) has been a prognostic tool for assessing the potential effects of formulation on the human drug oral bioavailability. When used in conjunction with in vitro dissolution tests, the BCS can support the prediction of in vivo product performance and the development of mechanistic models that support formulation assessments through the generation of “what if” scenarios. To date, the applicability of existing human BCS criteria has not been evaluated in dogs, thereby limiting its use in canine drug development. Therefore, we examined 50 drugs for which absolute bioavailability ( F) was available both in dogs and humans. Adobe flash cc 2014 crack.
The drugs were also evaluated for any potential association between solubility (calculated from the dose number, Do) or lipophilicity (LogP) and F in dogs. In humans, solubility is determined in 250 mL of fluid. However, the appropriate volume for classifying drug solubility in dogs has not been established. In this analysis, the estimated volume of a water flush administered to fasted dogs (6 mL) and a volume of 250 mL scaled to a Beagle dog (35 mL) were examined. In addition, in humans, a Do value greater than 1.0 is used to define a compound as highly soluble and a LogP value greater than 1.72 as high permeability. Download x plane 10 demo. These same criteria were applied for defining highly soluble and highly permeable in dogs. Fl studio reg key generator.
College Pharmaceuticals
Whether using 35 or 6 mL to determine Do, the canine solubility classification remained unchanged for all but seven compounds. There were no clear associations between a drug’s F in dogs and humans or between the canine value of F and either its human BCS classification, its LogP value, or the canine Do estimate. There was a tendency for those drugs with canine values of F equal to or greater than 80% to have LogP values equal to or greater than 1.0. Exceptions to this observation tended to be those compounds known to be absorbed via mechanisms other than passive diffusion (e.g., via transporters or paracellular transporters). Although there are limitations to the approach used in this study, the results of our assessment strongly suggest that the human BCS classification system requires substantial modification before it can be reliably applied to dogs. The United States Pharmacopeial Convention (USP) authorized the creation of an advisory panel to investigate the possibility of applying the principles of the Biopharmaceutics Classification System (BCS) to veterinary drugs—specifically, solid oral formulations administered to dogs ( ). Developed for human pharmaceutical compounds (,,,, ), the BCS is an important tool that facilitates product development and regulatory decisions.





